Global Journal of Cancer Therapy
ANXA2 Enhances the Malignancy of Gastric Cancer Cells by Remodeling Cytoskeleton
Yujuan Zhou1, Wanlin Che1, Xue Bai1, Danying Zhang1, Wenxuan Zhang1, Hongming Fang1, Guochao Nie2* and Yingchun Hou1*
1College of Life Sciences, Shaanxi Normal University, Xi’an, Shaanxi 710119, China
2Guangxi Key Laboratory of Agricultural Resources Chemistry and Biotechnology, Guangxi Colleges and Universities Key Laboratory of Efficient Utilization of Special Resources in Southeast Guangxi, College of Chemistry and Food Science, Yulin Normal University, Yulin 537000, China
Dr. Guochao Nie, Guangxi Key Laboratory of Agricultural Resources Chemistry and Biotechnology, Guangxi Colleges and Universities Key Laboratory of Efficient Utilization of Special Resources in Southeast Guangxi, College of Chemistry and Food Science, Yulin Normal University, Yulin 537000, China, E-mail: bccu518@163.com
Cite this as
Zhou Y, Che W, Bai X, Zhang D, Zhang W, Fang H, et al. ANXA2 Enhances the Malignancy of Gastric Cancer Cells by Remodeling Cytoskeleton. Glob J Cancer Ther. 2024;10(1):001-008. Available from: 10.17352/2581-5407.000053Copyright License
© 2024 Zhou Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.ANXA2 was reported as a multiple tumors relevant gene expressed excessively in many tumors, especially in cancers from the digestion system, and its aberrant expression enhances the malignant properties of cancer cells. We suppose that microstructure heterogeneity is important to maintain the malignancy of cancer cells, and ANXA2 excessive expression enhances the malignancy by remodeling the microstructures of cancer cells. To validate the proposal, we studied the effects of ANXA2 expression on the remodeling of motility-associated microstructures in SGC-7901 cells by a series of morphological display methods. The results showed that the cell motility microstructures, such as pseudopodia/filopodia and microvilli, were weaker and poorer in ANXA2-silenced SGC-7901 cells than in wild type cells, and the malignancy was significantly decreased in ANXA2 silenced SGC-7901 cells. These findings indicate that ANXA2 is necessary to maintain the changed cytoskeleton of cancer cells, especially the highly polymerized cytoskeleton protein, actin, and tubulin, which leads to the alteration of the cell microstructures that are closely correlated with cell motility. Our results indicate that ANXA2 plays an important role in enhancing the malignancy of gastric cancer cells by remodeling the cytoskeleton microstructures of polymerization. In this short article, we will try to shed light on this very important problem helping chronic pain patients to understand the nature of their pain and advising them how to deal with it.
Gastric cancer (GC) is the fifth most common cancer in the digestive system and the third cause of cancer death globally [1]. Despite the promise of cancer medicine, major difficulties currently confront the treatment of cancer patients by chemotherapy resistance and recurrence [2]. ANXA2 (Annexin A2) is a multifunctional Ca2+ and phospholipid-binding protein that is expressed in a wide spectrum of cells, including those participating in the inflammatory response [3]. ANXA2 is a 36-kDa member of the annexin family that is a Ca2+ binding phospholipid protein family involved with tumor development, and involved in endocytosis, exocytosis, membrane domain organization, actin remodeling, signal transduction, protein assembly, transcription and mRNA transport, as well as DNA replication and repair [4]. Published and our previous data indicate that the interactions of ANXA2 with other proteins function to maintain the tumor microenvironment and enhance tumor growth [5].
The special appearances and structures, especially microstructures of cancer cells are usually changed as some morphological heterogeneity compared with normal cells. The cytoskeleton is the major mechanical structure of the cell, and dynamically polymerized skeleton comprised of microtubules, actin, and intermediate filaments [6]. ANXA2 expression inhibition in HCC cells caused cancer cell malignancy to improve, which may benefit HCC therapy [7].
To study the significance of ANXA2 on the malignancy of GC cells by remodeling motility-associated microstructures, we silenced the expression of ANXA2 in SGC-7901 cells and then detected or displayed the changes in the microstructure, especially the cytoskeleton.
Materials and methods
Cell culture
The SGC-7901 cell line was purchased from ATCC (Rockville, MD). The cells were cultured in DMEM medium purchased from Invitrogen (New York, NY) supplemented with 10% fetal bovine Serum (FBS), 100U/ml penicillin, and 50 μg/ml streptomycin at 37 0C in a humidified incubator with 5% CO2. Cells in the exponential phase of growth were used in each experiment.
siRNA vector construction and transfection
ANXA2 targeting siRNAs was designed following the description by Vert, et al [8]. The vectors of targeting siRNAs and siRNAscr (negative control) were constructed using pU6H1-GFP by Biomics Biotech (Nantong, China). siRNAs were induced into SGC-7901 cells using the calcium phosphate transfection method described by Mostaghaci, et al [9]. The medium was replaced with the medium containing antibiotics at 16 h post-transfection.
RT-PCR analysis
To confirm the inhibition efficiency of the siRNAs to ANXA2 at mRNA level, the transcriptome of the cells siRNA transfected at 72 h post-transfection were isolated using a Biozol reagent kit (BIOER, Hangzhou, China) following the manufacture instructions, and then RT-PCR was used to evaluate the inhibition efficiency.
Western blot analysis
A 10% SDS-PAGE was run, and the lysate containing 20 μg protein was loaded into each lane unless stated otherwise. The gel was transferred onto the NC membrane (PALL, Washington, USA) at 40C overnight, blocked with 4.5% fat-free milk in TBST buffer containing 0.1% tween-20 (TBST) for 1 h, incubated overnight at 40C with rabbit anti-ANXA2 and GAPDH purchased from Santa Cruz Biotechnology, Inc. or Cell Signaling Technology, Inc. (CA, USA) (1:300 and 1:500.), washed 3 times in TBST, re-incubated with HRP-conjugated Goat anti-rabbit (1:3000. Bioss, Beijing, China) for 2 h at room temperature, re-washed 3 times in TBST, and finally developed using enhanced chemiluminescence (ECL) kit (Pierce, Shanghai, China).
MTT assay
MTT assay was used to detect the cell proliferation. 3 × 104cells/well were seeded into a 96-well plate, the lysate of each group was prepared at 24 h, 48 h, 72 h and 96 h post-transfection using 20 μl MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide](Sigma, USA) at 5 mg/ml working concentration, and incubated at 370C for 5rs, finally added 150 μl dimethyl sulfoxide (DMSO) into each well and incubated for 10 mins at room temperature to dissolve formazan crystals. The absorbance of each well was measured with an ELISA reader (BIO-TEK, New York, USA) at 562 nm. The DMSO-treated group was used as a control.
Wound healing assay
Wound healing assay is a popularly used method to estimate cell motility. When the transfected cells were cultured to subconfluent density (80%) in a 30 mm dish, a hole wound area was created with a pipette tip linked to a pump. The hole wound area was photographed at 0 h, 24 h, and 48 h post hole making, and measured using a software, Gene Tools, supplied with a gel imager together (Junyi, Beijing, China). The cell motility (migration rate) was evaluated using the formula as follows: migration rate = (1 − At/A0) × 100%. A0 = the wound area at 0 h, and At = wound area at 24 h or 48 h.
Transwell chamber assay
Briefly, cells were seeded into the top chamber with 8μm pores (Corning Costar Incorporated, New York, NY, USA) by 8 × 103 cells/200 μL serum-free medium, and the bottom chamber was filled with RPMI1640 medium supplemented with 10% FBS. The top chamber membrane was fixed with methanol, stained with 0.1% crystal violet solution, then imaged and counted under an inverted optical microscope.
Coomassie brilliant blue staining
Cells were cultured on coverslips in 30 mm dishes, transfected using the same protocol as above, fixed in 4% paraformaldehyde at 72 h post-transfection, permeabilized with 0.1% triton X-100, and finally stained with 0.2% coomassie brilliant blue R-250 (Dingguo, Xi’an, China) for45mins at room temperature. Slips were imaged under a fluorescence microscope (Zeiss, Germany).
Observation of the microstructure changes
Scanning Electron Microscope (SEM): Cells were cultured on the coverslip in 30 mm dishes for 72 h after transfection. The slip was rinsed using PBS, fixed in 2.5% glutaraldehyde(Sigma, St. Louis, NY) for 1 h at 4 0C, dehydrated ingredient diluted ethanol solutions (30%, 50%, 70%, 80%, 90%, 95%, 100%), and finally imaged under a SEM (Quanta2000, Philips-FEI, Netherlands) following the instruction book supplied with this microscope together.
Transmission Electron Microscope (TEM): The slips’ treatment was the same as above and imaged under a TEM (JEM-2100, Japan) following the instruction book supplied with this microscope together.
Display of the microstructure-associated proteins
The slip treatment was the same as above and incubated in 2% BSA for 1 h. The treated slips were respectively incubated with FITC-labeled phalloidine (1:200) and the rabbit anti-human antibodies against β-tubulin or Lamin B (1:50) in a blocking buffer overnight at 40C. After washing 3 times in PBS, the slips incubated respectively with Cy3-labeled goat-anti-rabbit for β-tubulin display, HRP goat anti-rabbit for Lamin B display, 49-6-Diamidino-2-phenylindole (DAPI, Roche, Germany) or Hoechst 33342 (kaiji, Nanjing, China) diluted in blocking buffer (1:500) for 2 h for nuclei display. Finally, the slips were imaged under the fluorescence microscope or laser scanning confocal microscope (TCS SP5, Leica, Germany).
Statistical analysis
The statistical software, SPSS13.0, was used for all statistical analyses. Each grouped experiment was repeated 3 times, and the result data averages of each group were compared to each other and shown as M ± SD.
Ethical considerations
This study did not involve any sample of tissue from humans or animals.
Results
The inhibition of ANXA2 expression suppressed the development of motility-associated microstructures in gastric cancer cells
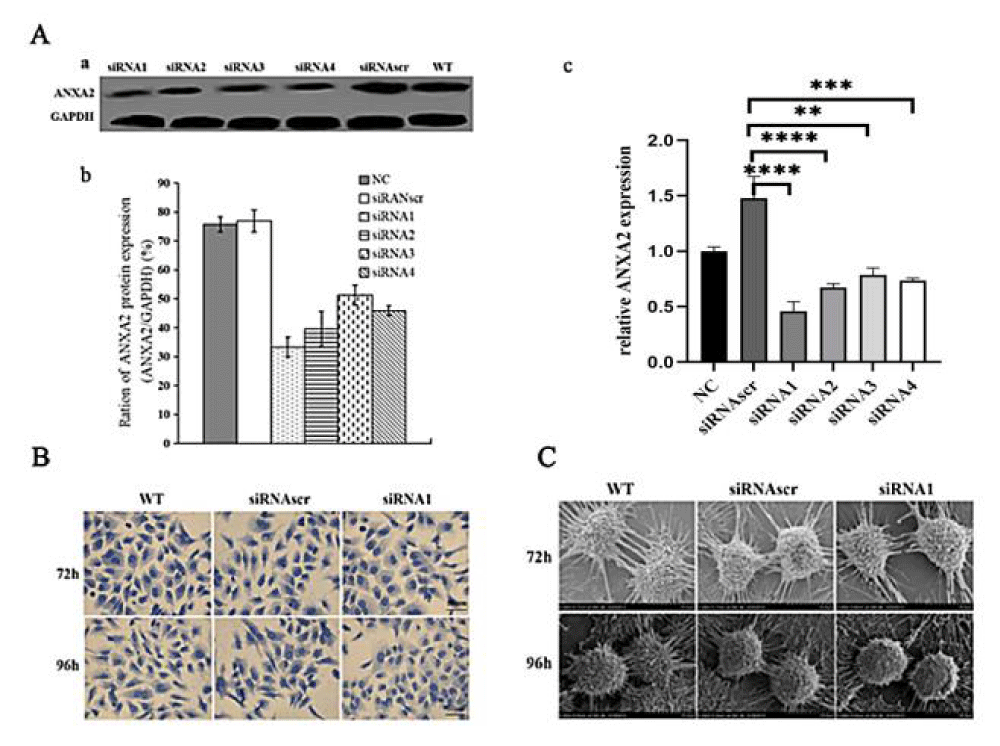
To evaluate the inhibition efficiency of the siRNAs to ANXA2, the Western blot and RT-PCR were used to detect ANXA2 expression at protein and mRNA levels. Results indicated that ANXA2 expression was significantly inhibited at levels of protein ((Figure 1A a,b) by 70% (siRNA1, p < 0.05) and mRNA (Figure 1A c) by siRNA1 (The continued experiments used siRNA1 only).
The pseudopodia/filopodia of each group were observed by Coomassie brilliant blue staining or under scanning electron microscopes, their developments in the siRNA1 group were significantly inhibited and contact inhibition enhanced (Figure 1B, C). These findings indicate that ANXA2 expression is important to maintain cell motility for enhancing invasion and metastasis of cancer cells.
The inhibition of ANXA2 expression remodeled the cytoskeleton by alternating the polymerization of F-actin and β-tubulin in gastric cancer cells
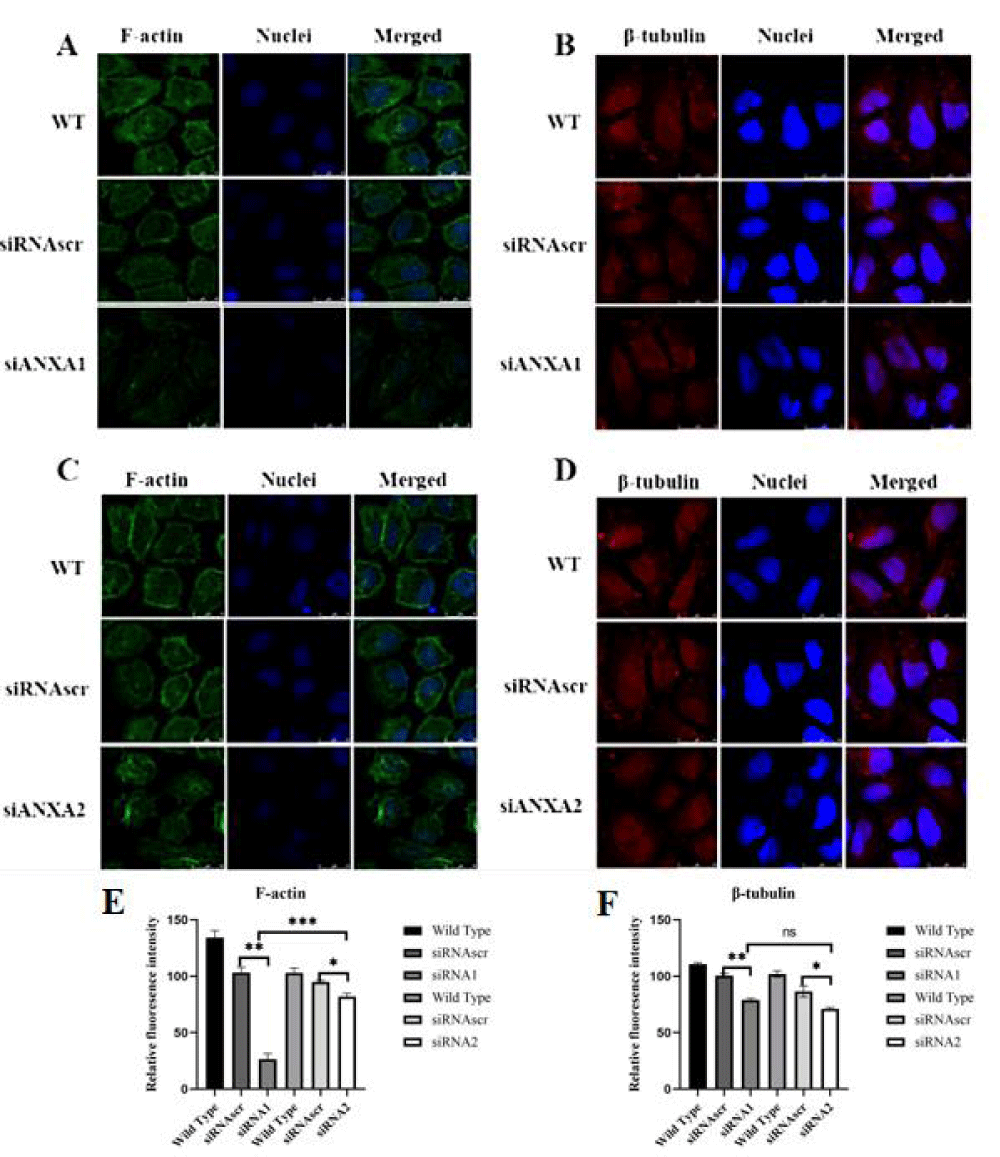
Any effect on F-actin bundle assembly or disassembly may be important to regulate the motility, invasion, and metastasis of cancer cells. Using FITC-labeled phalloidine staining, F-actin expression was significantly enhanced and vigorously bundled and polymerized along the inner face of the membrane in control group cells, but distinctly inhibited and disassembled in the cells of the interfered group (Figure 2A,C), the results above indicate that ANXA2 enhances the polymerization of F-actin in SGC-7901 cells.
Microtubule is one of the exoskeleton basic proteins associated with cell proliferation, motility, and material transportation, so it is closely relevant to the malignancy maintenance of cancer cells. The polymerization of β-tubulin was moderately inhibited in the interfered group (Figure 2B,D), and no significant difference between both control groups. This result implies that ANXA2 may enhance the microtubule assembly for microtentacles formation in cancer cells to sustain their proliferation and metastasis potentials.
The inhibition of ANXA2 expression suppressed the malignancy in gastric cancer cells
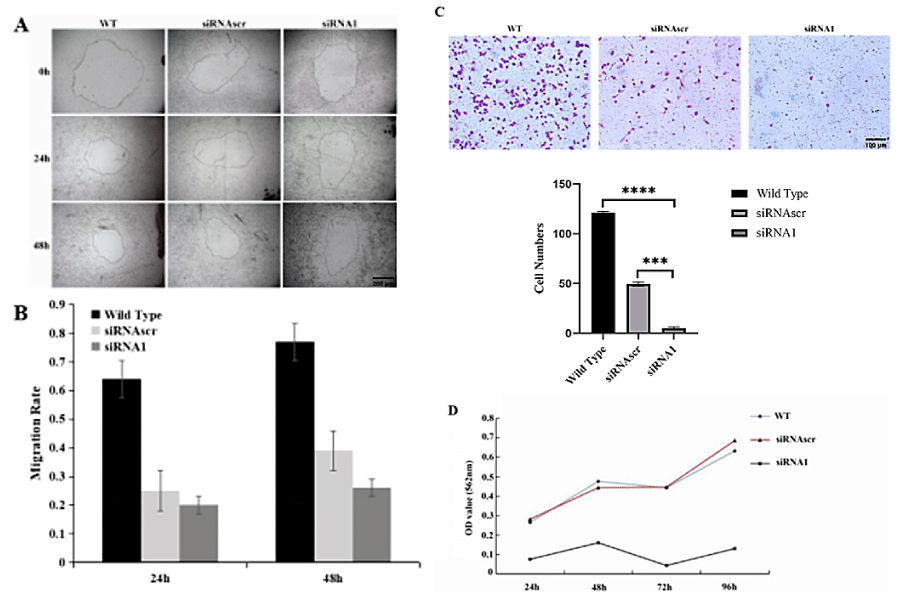
Down-regulation of ANXA2 inhibited cell proliferation: High or active motility is an important malignant phenotype of cancer cells. We used migration rate to evaluate cell motility and designated a hole wound area healing assay (Figure 3A, B) and a transwell assay (Figure 3C) for it. The results indicated that down-regulation of ANXA2 expression inhibited cell migration significantly.
MTT assay was used to evaluate the cell proliferation of ANXA2 down-regulated cells. The result explored that the proliferation of ANXA2 down-regulated cells was significantly suppressed (Figure 3D).
Discussion
Epithelial or endothelial cancer cells metastasize in a series of linked, sequential steps initiated by extracellular matrix remodeling followed by local tumor invasion. Elucidation of the molecular processes contributing to an invasive cell phenotype is critical to understanding tumor cell metastasis [10]. The cancer cell motility enhancement of ANXA2 is possibly programmed by activated EGFR-Src [11], JNK/c-Jun [12], NF-κB [13], and Akt1-mTOR-ULK1/2 [14] signal pathways for the increased expression and polymerization of cell microstructure associated proteins, such as F-actin [15] and β-tubulin [16].
Our results showed that the inhibited expression of ANXA2 induced the decreased polymerization of F-actin and β-tubulin, particularly for F-actin (Figure 2), subsequently the weak motility associated plasticity of cancer cell dynamic cytoskeleton modeled by the polymerization of F-actin and β-tubulin following the increased cell contact inhibition (Figure 1), an important phenotype of cancer cell malignancy, and caused a dramatic effect on cells properties with statistically significant reduction of cell migration and proliferation (Figure 3). The subsequently inhibited expression of F-actin and β-tubulin resulted in the poor development of cell pseudopodia/filopodia with suppressed contact inhibition (Figure 1), and finally led to the inhibited malignancy of cancer cells. Our results are also supported by previous publications [17-20].
ANXA2 was reported as a malignant enhancer of GC cells [16], and it is possibly intermediated by the activated RhoAEGFR-Src, JNK/c-Jun [21] or Rho/ROCK/LIMK/Cofilin [22] signal pathways [23]. Our previous study [24] reported that ANXA2 changes GC cell malignancy as a cancer enhancer, but current study further focused on ANXA2 critical role in maintaining the malignancy in GC cells by remodeling motility-associated microstructures, especially in enhancing cell motility by remodeling cytoskeleton in GC cells.
In summary, our results explored that ANXA2 plays important roles in maintaining the enhanced malignancy of GC cells by enhancing the polymerization of F-actin and β-tubulin, especially F-actin, and subsequently causes the cell motility associated microstructures and cytoskeleton remodeled for the vigorously enhanced malignancy of GC cancer cells. Therefore, ANXA2 can be considered as a potential bio-marker or target candidate for the diagnosis and therapy of GC in the future.
Declarations
Availability of data and materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Funding: This work was funded by the Guangxi Innovation Driven Development Major Project (No. Guike AA20302013) to G Nie. The APC was funded by this grant.
Authors’ contributions: YJZ wrote the main manuscript and performed the statistical analysis. YCH provided conceptualization and methodology. GCN provided funding. HMF, XB, DYZ, WXZ, and WLC handled experiments and data assay. All authors reviewed and approved the final manuscript.
- Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635-648. Available from: https://doi.org/10.1016/s0140-6736(20)31288-5
- Kim J, Cheong JH. Role of mitochondria-cytoskeleton interactions in the regulation of mitochondrial structure and function in cancer stem cells. Cells. 2020;9(7):1691. Available from: https://doi.org/10.3390/cells9071691
- Dallacasagrande V, Hajjar KA. Annexin A2 in inflammation and host defense. Cells. 2020;9(6):1499. Available from: https://doi.org/10.3390/cells9061499
- Grindheim AK, Saraste J, Vedeler A. Protein phosphorylation and its role in the regulation of annexin A2 function. Biochim Biophys Acta Gen Subj. 2017;1861(11 Pt A):2515-2529. Available from: https://doi.org/10.1016/j.bbagen.2017.08.024
- Chaudhary P, Thamake SI, Shetty P, Vishwanatha JK. Inhibition of triple-negative and Herceptin-resistant breast cancer cell proliferation and migration by annexin A2 antibodies. Br J Cancer. 2014;111(12):2328-2341. Available from: https://doi.org/10.1038/bjc.2014.542
- Pegoraro AF, Janmey P, Weitz DA. Mechanical properties of the cytoskeleton and cells. Cold Spring Harb Perspect Biol. 2017;9(11). Available from: https://doi.org/10.1101/cshperspect.a022038
- Shi H, Xiao L, Duan W, He H, Ma L, Da M, Duan Y, Wang Q, Wu H, Song X, Hou Y. ANXA2 enhances the progression of hepatocellular carcinoma via remodeling the cell motility associated structures. Micron. 2016;85:26-33. Available from: https://doi.org/10.1016/j.micron.2016.03.008
- Vert JP, Foveau N, Lajaunie C, Vandenbrouck Y. An accurate and interpretable model for siRNA efficacy prediction. BMC Bioinformatics. 2006;7:520. doi: 10.1186/1471-2105-7-520. Available from: https://bmcbioinformatics.biomedcentral.com/articles/10.1186/1471-2105-7-520
- Mostaghaci B, Loretz B, Lehr CM. Calcium phosphate system for gene delivery: historical background and emerging opportunities. Curr Pharm Des. 2016;22(11):1529-1533. Available from: http://dx.doi.org/10.2174/1381612822666151210123859
- Cammarota F, Laukkanen MO. Mesenchymal stem/stromal cells in stromal evolution and cancer progression. Stem Cells Int. 2016;2016:4824573. Available from: https://doi.org/10.1155/2016/4824573
- de Graauw M, Cao L, Winkel L, van Miltenburg MH, le Dévédec SE, Klop M, et al. Annexin A2 depletion delays EGFR endocytic trafficking via cofilin activation and enhances EGFR signaling and metastasis formation. Oncogene. 2014;33(20):2610-2619. Available from: https://doi.org/10.1038/bjc.2014.542
- Feng X, Liu H, Zhang Z, Gu Y, Qiu H, He Z. Annexin A2 contributes to cisplatin resistance by activation of JNK-p53 pathway in non-small cell lung cancer cells. J Exp Clin Cancer Res. 2017;36(1):123. Available from: https://jeccr.biomedcentral.com/articles/10.1186/s13046-017-0594-1
- Wang Y, Cheng YS, Yin XQ, Yu G, Jia BL. Anxa2 gene silencing attenuates obesity-induced insulin resistance by suppressing the NF-κB signaling pathway. Am J Physiol Cell Physiol. 2019;316(2). Available from: https://doi.org/10.1152/ajpcell.00242.2018
- Li R, Tan S, Yu M, Jundt MC, Zhang S, Wu M. Annexin A2 regulates autophagy in Pseudomonas aeruginosa infection through the Akt1-mTOR-ULK1/2 signaling pathway. J Immunol. 2015;195(8):3901-3911. Available from: https://doi.org/10.4049/jimmunol.1500967
- Ju M, Ioannidou S, Munro P, Rämö O, Vihinen H, Jokitalo E, et al. A Na,K-ATPase-fodrin-actin membrane cytoskeleton complex is required for endothelial fenestra biogenesis. Cells. 2020;9(6):1387. Available from: https://doi.org/10.3390/cells9061387
- Montalvão F, Nascimento DO, Nunes MP, Koeller CM, Morrot A, Lery LMS, et al. Antibody repertoires identify β-tubulin as a host protective parasite antigen in mice infected with Trypanosoma cruzi. Front Immunol. 2018;9:671. doi: 10.3389/fimmu.2018.00671. Available from: https://doi.org/10.3389/fimmu.2018.00671
- He H, Xiao L, Cheng S, Yang Q, Li J, Hou Y, et al. Annexin A2 enhances the progression of colorectal cancer and hepatocarcinoma via cytoskeleton structural rearrangements. Microsc Microanal. 2019;25(4):950-960. Available from: https://doi.org/10.1017/s1431927619000679
- Varyukhina S, Lamazière A, Delaunay JL, de Wreede A, Ayala-Sanmartin J. The Ca2+- and phospholipid-binding protein annexin A2 is able to increase and decrease plasma membrane order. Biochim Biophys Acta Biomembr. 2022;1864(1):183810. doi: 10.1016/j.bbamem.2021.183810. Available from: https://doi.org/10.1016/j.bbamem.2021.183810
- Prislusky MI, Lam JGT, Contreras VR, Ng M, Chamberlain M, Pathak-Sharma S, et al. The septin cytoskeleton is required for plasma membrane repair. EMBO Rep. 2024;25(9):3870-3895. Available from: https://doi.org/10.1101/2023.07.12.548547
- Bărar AA, Pralea IE, Maslyennikov Y, Munteanu R, Berindan-Neagoe I, Pîrlog R, et al. Minimal change disease: pathogenetic insights from glomerular proteomics. Int J Mol Sci. 2024;25(11):5613. Available from: http://dx.doi.org/10.3390/ijms25115613
- Li Z, Zhang L, Gao M, Han M, Liu K, Zhang Z, et al. Endoplasmic reticulum stress triggers xanthoangelol-induced protective autophagy via activation of JNK/c-Jun axis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):8. Available from: https://doi.org/10.1186/s13046-018-1012-z
- Hitsuda A, Dan R, Urakawa A, Hiraoka Y, Murakami C, Yamamoto H, et al. 25-Hydroxycholesterol-induced cell death via activation of ROCK/LIMK/cofilin axis in colorectal cancer cell spheroids. J Steroid Biochem Mol Biol. 2022;216:106037. Available from: https://doi.org/10.1016/j.jsbmb.2021.106037
- McArthur S, Juban G, Gobbetti T, Desgeorges T, Theret M, Gondin J, et al. Annexin A1 drives macrophage skewing to accelerate muscle regeneration through AMPK activation. J Clin Invest. 2020;130(3):1156-1167. Available from: https://doi.org/10.1172/jci124635
- Sun MY, Xing RH, Gao XJ, Yu X, He HM, Gao N, et al. ANXA2 regulates the behavior of SGC-7901 cells. Asian Pac J Cancer Prev. 2013;14(10):6007-6012. Available from: https://doi.org/10.7314/apjcp.2013.14.10.6007

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
